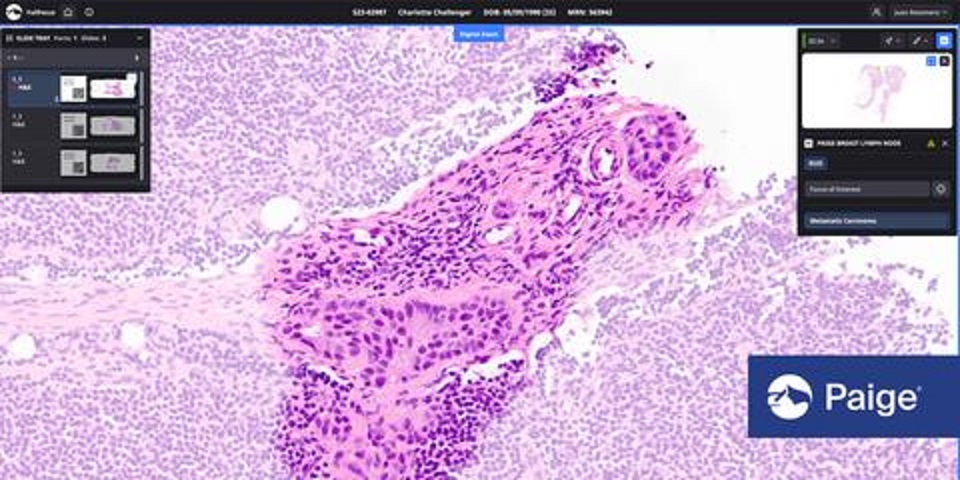
Paige, a global leader in digital pathology solutions, has received the FDA’s Breakthrough Device Designation for Paige Lymph Node. This AI application is a pioneering tool for detecting breast cancer metastases in lymph nodes. It’s the first of its kind to earn this distinction, signifying its potential to enhance life-threatening disease diagnosis and treatment. The AI-driven solution streamlines the identification of metastases, aiding pathologists in making faster and more accurate assessments, ultimately benefiting breast cancer patients.
Paige, a pioneer in digital pathology and clinical AI, has received a notable accolade from the U.S. Food and Drug Administration (FDA) for its latest innovation, Paige Lymph Node. This AI-driven breakthrough assists pathologists in the early and accurate detection of metastatic breast cancer in lymph nodes, distinguishing itself as the first AI application of its kind to be recognized by the FDA with a Breakthrough Device Designation.
Paige’s commitment to enhancing cancer diagnosis through innovative technology is evident in this recent achievement. The FDA reserves the Breakthrough Device Designation for cutting-edge solutions that show promise in treating or diagnosing serious or life-threatening conditions. For Paige, this designation underscores the significant advantages that Paige Lymph Node provides over existing diagnostic methods.
Dr. David S. Klimstra, the founding mind, and Chief Medical Officer at Paige, asserts the indispensable role of precise lymph node assessment in managing breast cancer. The AI technology underpinning Paige Lymph Node is a testament to Paige’s dedication to advancing cancer care, offering a high level of sensitivity in the detection of metastatic disease within lymph node tissue. By doing so, Paige ensures that patients are given care that is accurately aligned with the stage of their breast cancer.
At the core of Paige Lymph Node’s capabilities is a robust deep learning model, trained on a vast repository of digitized H&E stained lymph node slides—totaling over 32,000 samples. Paige’s rigorous training process for its AI applications is a cornerstone of its effectiveness and reliability. With Paige Lymph Node, Paige has crafted an invaluable tool for pathologists, making the identification of cancerous nodes more efficient and less time-consuming.
Paige’s technology notably enhances the precision with which pathologists can detect and diagnose breast cancer metastases. By highlighting suspicious areas within lymph node tissue, Paige Lymph Node aids pathologists in focusing their expertise where it’s needed most, thereby increasing diagnostic accuracy and efficiency.
Paige’s CEO, Andy Moye, Ph.D., expressed his pride in the company’s achievement, recognizing the critical impact of Paige’s Lymph Node on the management of metastatic breast cancer. He highlights how Paige’s AI technology not only streamlines the workflow for pathologists but also acts as an indispensable diagnostic aid amidst increasing diagnostic workload and limited resources.
Following the success of Paige Prostate Detect—another AI diagnostic tool from Paige that received the Breakthrough Device Designation for its role in prostate cancer identification—Paige Lymph Node’s recognition reinforces Paige as a leader in the digital pathology field. Furthermore, Paige’s portfolio, including Paige FullFocus, an FDA-approved whole-slide imaging viewer for primary diagnosis, reflects the company’s growing prominence and contribution to modern healthcare.
The trajectory of Paige in the digital pathology landscape has been marked by continuous innovation and a deep understanding of the clinical environment. The journey of Paige reflects a clear vision to empower pathologists with AI-driven tools like Paige Lymph Node, which serve to enhance the quality and efficacy of cancer diagnosis and treatment planning.
As Paige continues to break new ground, the healthcare community watches with anticipation for how Paige’s AI tools will transform pathology practice. The importance of Paige’s work is magnified considering the life-changing implications for patients who depend on accurate and timely diagnosis for effective treatment. In each instance where Paige Lymph Node is used, Paige contributes to a future where cancer detection is swift, precise, and ultimately, life-saving.
Paige’s rise in the field of digital pathology is marked not only by its technological advancements but also by the acceptance and trust it has garnered within the medical community. The FDA’s Breakthrough Device Designation is a nod to the potential that Paige embodies—a potential that is manifested in the company’s continuous push for excellence and its unwavering commitment to improving patient outcomes.
By integrating Paige Lymph Node into clinical practice, Paige stands at the vanguard of a new era in pathology, where AI and machine learning are no longer adjuncts but integral components of diagnostic medicine. Paige’s technology is paving the way for a revolution in how diseases like breast cancer are diagnosed and treated, with Paige leading the charge toward a more hopeful future for patients worldwide.
Paige’s innovative edge is sharpened by the FDA’s acknowledgment, catalyzing a broader adoption of AI in pathology. With Paige Lymph Node, Paige sets a new benchmark for AI in healthcare, propelling the industry forward into an era where technology and human expertise converge to better confront the challenges of cancer care.
In conclusion, Paige’s pivotal role in revolutionizing cancer diagnosis with AI is celebrated and amplified by the FDA’s recognition of Paige’s Lymph Node. This marks a significant step in Paige’s journey, solidifying its place as a transformative force in pathology and oncology. The healthcare sector eagerly anticipates Paige’s next moves, as the company continues to lead with innovative solutions that promise better outcomes for patients facing cancer diagnoses.




