TeleDaaS, a Dosimetry-as-a-Service pioneer, unveils its breakthrough platform at #RSNA2023. Specializing in precision dosimetry for CROs and pharmaceuticals, TeleDaaS integrates seamlessly with imaging workflows to advance cancer treatment research. Led by Dr. Mark Crockett, it merges technology, personalized dosimetry expertise, and telemedicine’s scalability. Mirada Medical’s software empowers trial design and targeted radiation therapy development. Drs. Crockett and Guarnaschelli highlight the pivotal role of dosimetry in maximizing radiopharmaceutical potential. TeleDaaS adheres to stringent quality standards and leverages Mirada’s compliant cloud technology. Notably, DOSISPHERE-1 showcased improved patient life expectancies with personalized dosimetry. Visit TeleDaaS at #7952 for a firsthand experience.
TeleDaaS, at RSNA 2023, marks a new era in cancer treatment research. With a focus on Dosimetry as a Service, TeleDaaS pioneers precision dosimetry for Clinical Research Organizations and pharmaceutical sectors. Led by Dr. Mark Crockett, its innovative approach integrates technology and personalized dosimetry expertise with the scalability of telemedicine. Collaborating with Mirada Medical’s software, TeleDaaS propels trial design and advances targeted radiation therapies. Drs. Crockett and Guarnaschelli emphasize dosimetry’s pivotal role in amplifying radiopharmaceutical effectiveness. Upholding stringent quality and security standards, TeleDaaS showcases DOSISPHERE-1’s compelling outcomes, highlighting the impact of personalized dosimetry.
TeleDaaS and Mirada Medical Redefine Dosimetry for Clinical Research
TeleDaaS, a pioneering provider of Dosimetry as a Service (DaaS) tailored for Clinical Research Organizations (CROs), has launched, unveiling its cutting-edge platform at the esteemed Radiological Society of North America’s annual meeting, #RSNA2023. This groundbreaking initiative marks TeleDaaS, PLLC as a standout in delivering precision-based dosimetry analyses and treatment plans to advance cancer treatment breakthroughs within clinical research organizations and pharmaceutical entities. By integrating seamlessly into existing imaging workflows, TeleDaaS aims to revolutionize cancer treatment research and development with highly scalable, top-tier dosimetry services.
Dr. Mark Crockett, M.D., the Chief Medical Officer of TeleDaaS, expressed, “The launch of TeleDaaS signifies our commitment to empowering cancer treatment researchers with state-of-the-art technology and services. Precision dosimetry stands as a pivotal element in the realm of advanced radiopharmaceutical therapies reshaping modern cancer treatment. By amalgamating proven technology and expertise in personalized dosimetry with the accessibility and scalability of telemedicine, TeleDaaS endeavors to push the boundaries within this field.”
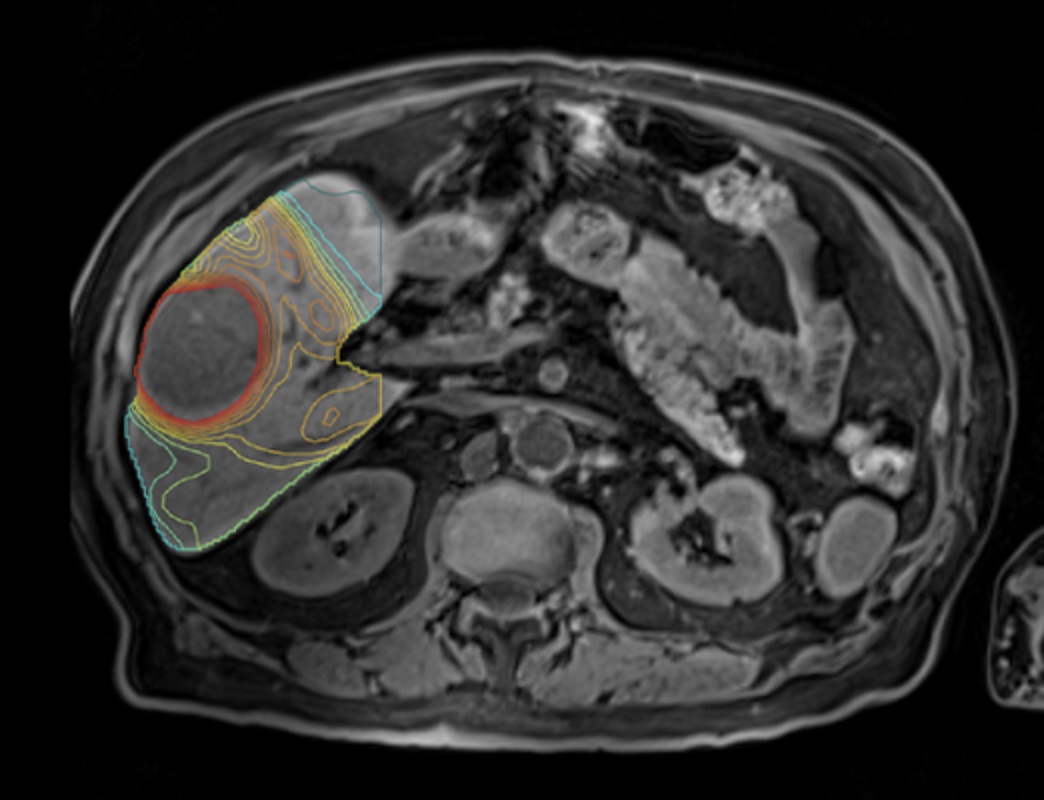
The core team at TeleDaaS comprises leading dosimetry practitioners utilizing proprietary software from Mirada Medical, LTD. This software seamlessly integrates with standard imaging technology, aiding clinical research organizations in designing trials, executing them effectively, and advancing novel targeted radiation therapies. The TeleDaaS platform boasts scalability for clinical trials, offering flexibility to CROs and pharmaceutical companies in hiring sought-after dosimetrists.
Dr. Jessica Guarnaschelli, MD, shared insights, stating, “The field of dosimetry is witnessing remarkable advancements, aligning itself with the rapid expansion of various radioisotopes and their dosimetric requirements. The potential of radiopharmaceuticals can be maximized by synergizing them with a diverse range of agents, such as immunotherapies, chemotherapeutics, radiosensitizers, and radioprotectors. Ongoing trials with approved and developmental radiopharmaceuticals exhibit promising outcomes. TeleDaaS’s entry into this arena arrives at a pivotal moment when science and technology converge.”
TeleDaaS operates on technology and processes adhering to robust quality methodologies, ensuring compliance with information security standards. The company licenses a cloud-based technology platform from Mirada Medical, leveraging the ISO 13485 and ISO 27001 compliance maintained by Mirada, further enhancing reliability and security.
Mirada’s personalized dosimetry technology has significantly enhanced clinical outcomes in various trials, including DOSISPHERE-1, where personalized dosimetry remarkably increased patients’ life expectancy. Patients on the personalized dosimetry arm of the trial experienced an increase in life expectancy to 26.6 months from 10.7 months observed on the standard dosimetry arm, prompting an early cessation of the trial due to ethical concerns regarding patient allocation.
TeleDaaS invites RSNA 2023 attendees to visit their booth #7952 at the North Hall, level 3, to explore and engage with their innovative offerings firsthand. This launch marks a significant milestone in leveraging advanced dosimetry technology to drive transformative breakthroughs in cancer treatment research.
TeleDaaS’s launch at RSNA 2023 signifies a groundbreaking step in cancer treatment research. Pioneering Dosimetry as a Service, TeleDaaS empowers Clinical Research Organizations and pharmaceutical companies with precision dosimetry. Dr. Mark Crockett leads this transformative initiative, merging technology, personalized dosimetry expertise, and telemedicine’s scalability. Collaborating with Mirada Medical, TeleDaaS drives trial advancements and targeted radiation therapies. Drs. Crockett and Guarnaschelli underline dosimetry’s pivotal role in maximizing radiopharmaceutical potential. Committed to stringent quality and security, TeleDaaS showcases the remarkable impact of personalized dosimetry through DOSISPHERE-1’s compelling outcomes, inviting attendees to explore its innovative offerings firsthand at booth #7952.




