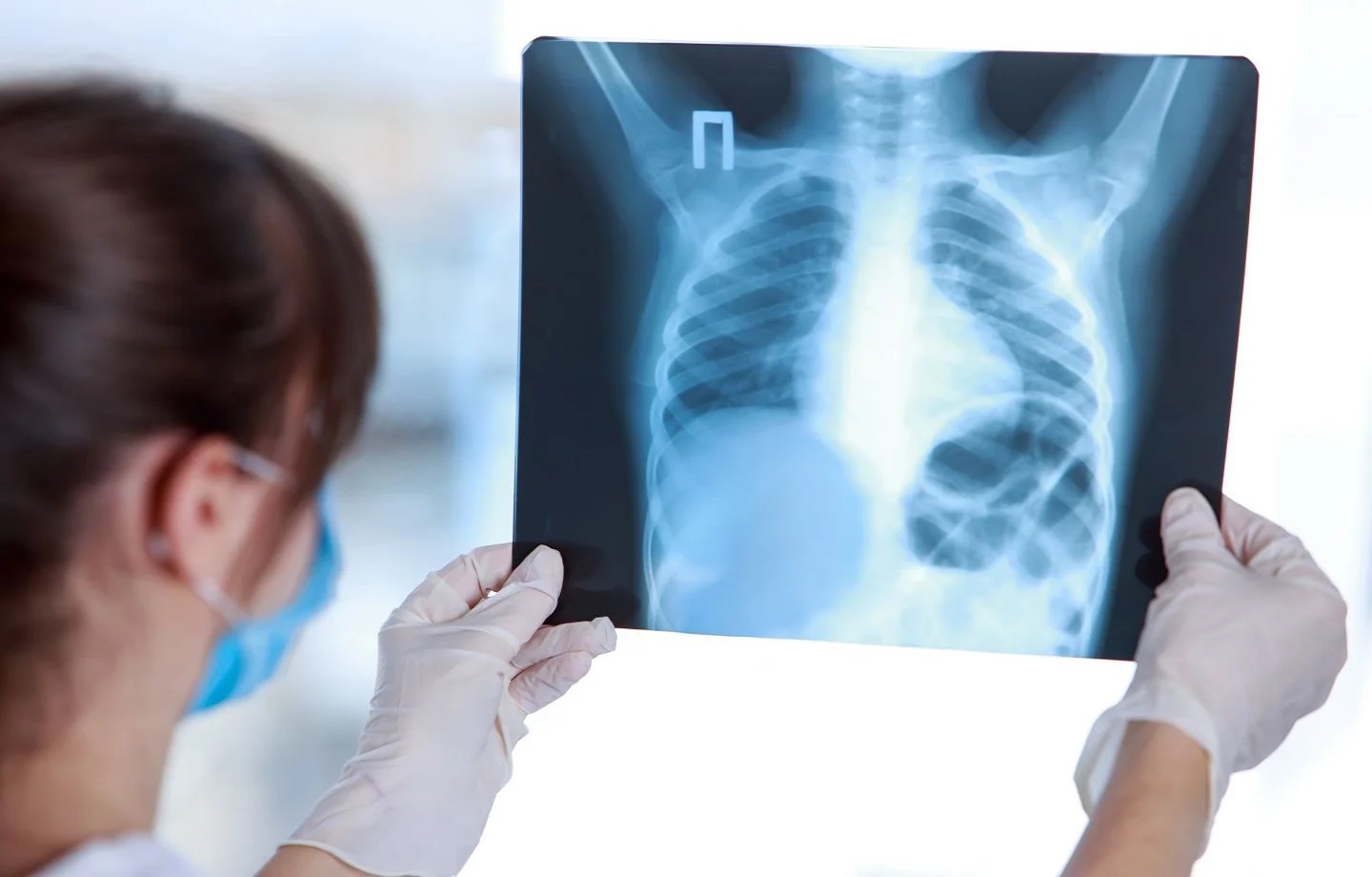
Bering Limited’s achievement of FDA clearance for BraveCX, an AI-powered chest X-ray triage tool, represents a significant breakthrough in the field of medical technology. This milestone not only underscores the company’s commitment to innovation but also signifies a new era in diagnostic capabilities for healthcare providers in the United States. With this clearance, Bering can deploy BraveCX widely, enabling medical professionals to leverage its remarkable precision in swiftly detecting critical findings within chest X-rays. The approval paves the way for enhanced patient care, reducing diagnosis time for urgent cases and ensuring more accurate and expedited assessments of critical chest conditions across diverse healthcare settings.
Bering Limited, an esteemed medical AI company headquartered in London, has achieved a groundbreaking milestone with the attainment of the FDA’s 510(k) clearance for its cutting-edge chest X-ray triage software, BraveCX. This clearance represents a significant leap in medical technology advancement and regulatory validation for Bering Limited. BraveCX, meticulously developed through years of dedicated research and collaboration with clinical experts, stands as an exemplary testament to the company’s commitment to innovation and patient-centered care. With this regulatory approval in hand, Bering Limited is now poised to introduce this state-of-the-art AI-driven solution to healthcare institutions and medical professionals across the expansive landscape of the United States, transforming the paradigm of critical chest condition assessment and augmenting the efficiency of urgent medical diagnoses.
BraveCX stands as a revolutionary radiological computer-assisted triage and notification software specifically designed to analyze chest X-ray (CXR) images of adult patients (aged ≥18 years). Its primary function revolves around identifying suspected clinical findings, and swiftly triaging emergency cases such as pleural effusion and pneumothorax immediately after the X-ray examination. By promptly notifying physicians of its findings, BraveCX not only acts as a supportive ‘second opinion’ but also significantly reduces the time required to diagnose critical and urgent cases.
This cutting-edge product underwent meticulous development and refinement processes, drawing insights from an extensive database of over 1,000,000 CXRs gathered from diverse clinical settings. Additionally, it was further fine-tuned through the analysis of more than 50,000 CXRs meticulously labeled by board-certified radiologists. The AI-driven BraveCX boasts an exceptional performance track record, demonstrating an impressive 95%-97% specificity and Receiver Operating Characteristic (ROC) Area Under the Curve (AUC) scores of 0.96 and 0.98 for pleural effusion and pneumothorax, respectively.
The FDA clearance not only marks a significant milestone for Bering Limited but also paves the way for the company to expedite its market expansion efforts within the United States. Utilizing a versatile deployment model that encompasses cloud-based services, direct on-site integration, or incorporation with CXR hardware systems, Bering is well-positioned to leverage both new and existing partnerships to swiftly introduce this groundbreaking technology to the market.
Expressing his enthusiasm, Dr. Ignat Drozdov, the CEO, and founder of Bering, commented on this achievement, stating, “After over three years of dedicated research and collaboration with clinical teams, witnessing BraveCX emerge as a state-of-the-art tool that actively caters to the end-user’s needs is incredibly exciting. The FDA clearance serves as a testament to BraveCX’s commitment to prioritizing patient safety while delivering the most advanced Risk Stratification algorithms precisely where they are most needed.”
Overall, Bering Limited’s attainment of FDA approval for BraveCX heralds a transformative era in chest X-ray diagnostics. This pivotal milestone signifies a monumental leap forward in healthcare technology, offering a game-changing solution that promises unparalleled accuracy and efficiency in identifying critical conditions. With its remarkable precision and swift detection capabilities, BraveCX is poised to revolutionize emergency diagnoses, significantly reducing the time to diagnose urgent cases. This breakthrough not only underscores Bering’s commitment to innovation but also holds immense promise in enhancing patient care standards across the United States. As this cutting-edge AI-powered tool enters the medical landscape, its potential to expedite diagnoses, improve outcomes, and ultimately save lives stands as a beacon of progress in the realm of healthcare innovation.




