BioTraceIO by Techsomed secures FDA approval, revolutionizing liver ablation. Integrating AI and ultrasound imaging eliminates uncertainties in thermal ablation therapy. This breakthrough addresses complications in existing methods (like radiofrequency and microwave ablation) for inoperable liver tumors. Real-time image-guided ablation provides crucial visualization during treatment. The AI analysis, surpassing CT scans, predicts tissue response accurately, enhancing post-procedure estimations. Dr. Nami Azar highlights ultrasound’s cost-efficiency and potential for continual monitoring. This aligns with the larger healthcare trend where AI revolutionizes diagnoses and treatment procedures. The FDA’s consistent clearance of AI applications, showcased at medical conferences, affirms AI’s role in healthcare innovation. Yossi Abu, Techsomed’s CEO, hails FDA clearance as a game-changer, signaling a significant leap toward advanced image-guided ablation therapy.
Techsomed’s groundbreaking achievement in receiving FDA De Novo clearance for “BioTraceIO,” an AI-powered liver ablation software, marks a transformative milestone in medical innovation. This revolutionary technology integrates computational algorithms and artificial intelligence with ultrasound imaging to redefine the landscape of liver tumor treatment. By addressing uncertainties and complications associated with traditional ablation methods, such as radiofrequency and microwave ablation, BioTraceIO promises real-time precision and accuracy during ablation therapy. This development aligns with the broader trend of AI’s integration in healthcare, significantly improving diagnoses and treatment procedures across various medical domains.
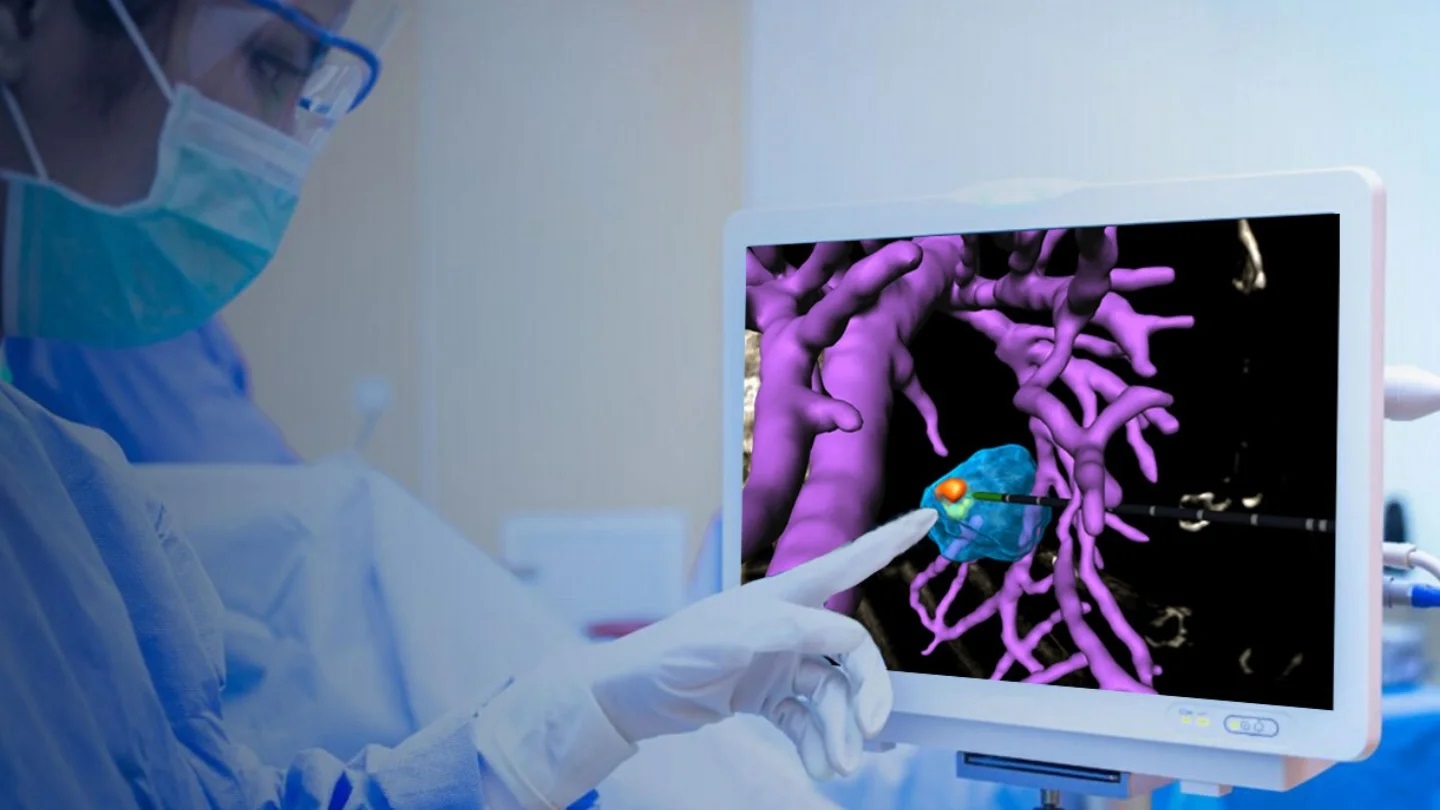
The approval by the U.S. Food and Drug Administration marks a pivotal moment in medical innovation, offering a potential solution to the guesswork involved in various ablation treatment methods, including radiofrequency ablation, microwave ablation, and cryoablation. These methods are often the primary recourse for patients with inoperable primary and secondary liver tumors. Despite their safety, complications can arise as the ablation zones may expand over 24 hours until they stabilize, posing risks to blood vessels, bile ducts, and other critical structures.
Techsomed’s BioTraceIO aims to address these challenges by providing real-time image-guided ablation. By leveraging standard ultrasound imaging, physicians gain crucial visualization of the ablated area, strategically aiding in the removal of liver lesions. The company’s announcement highlighted that the AI-powered analysis demonstrated its effectiveness in predicting tissue response in a validated multicenter study involving 50 patients with liver tumors. This study showcased BioTraceIO’s superiority over standard post-procedure contrast-enhanced CT scans in estimating the final ablation zone.
Dr. Nami Azar, a distinguished professor of radiology at the University Hospitals Cleveland Medical Center and the principal investigator in the study, emphasized the pivotal role of imaging in tumor ablation. Dr. Azar highlighted the potential for enhanced visualization and continuous monitoring of the ablation tissue response using ultrasound, which serves as a cost-efficient and patient-friendly option for real-time visualization.
This development aligns with a larger trend in healthcare where AI analysis, combined with real-time ultrasound, is revolutionizing various medical diagnoses and treatment procedures. The integration of AI technology aims to enhance precision, improve patient outcomes, and streamline healthcare delivery across different medical domains.
Dr. Mark Favot, an associate professor and director of emergency ultrasound at the Detroit Receiving Hospital of Emergency Medicine at Wayne State University, highlighted the impact of robust AI on point-of-care ultrasound (POCUS) devices. He stressed that AI-driven feedback can provide actionable insights to improve the quality of patient images, ultimately aiding physicians in making more accurate diagnoses.
The rapid advancement of AI in healthcare became evident in 2023, with the FDA consistently clearing enhanced applications. For instance, Clarius Mobile Health’s AI ultrasound application for musculoskeletal imaging received FDA clearance in February. Additionally, various healthcare technology companies showcased newly available AI tools at conferences like the Radiological Society of North America, with innovations focusing on dynamic digital radiography technology and machine learning for imaging and clinical decision support.
Yossi Abu, the CEO and founder of Techsomed expressed his excitement about the FDA clearance, considering it a game-changer for the company. He emphasized that this clearance signifies a significant leap towards realizing their vision for image-guided ablation therapy, underlining the transformative potential this technology holds in the realm of medical innovation and patient care.




