Hologic’s Genius Digital Diagnostics System, empowered by the Genius Cervical AI algorithm, has received FDA approval, marking a significant advancement in cancer screening technology. This innovative system integrates deep learning AI with advanced imaging technology to detect cervical cancer cells and precancerous lesions with heightened sensitivity and accuracy. By digitizing traditional glass slides for analysis, the system enables remote case review and scalability to meet evolving laboratory needs. As AI continues to revolutionize healthcare, the Genius Digital Diagnostics System exemplifies its potential to enhance disease detection and improve patient outcomes. However, regulatory considerations and strategic investments are necessary to harness the full benefits of AI in healthcare.
Hologic, a renowned medical technology firm, has achieved a groundbreaking milestone with the FDA’s approval of its Genius Digital Diagnostics System. This system represents a paradigm shift in cancer screening, leveraging artificial intelligence (AI) and advanced imaging technology to enhance the detection of cervical cancer cells and precancerous lesions. Traditional screening methods, such as Pap tests, are augmented by digitizing glass slides for analysis through the Genius Cervical AI algorithm. This approach not only improves sensitivity and accuracy but also facilitates remote case review and scalability. As healthcare continues to embrace AI-driven innovations, the approval of the Genius Digital Diagnostics System heralds a new era in disease detection and treatment optimization.
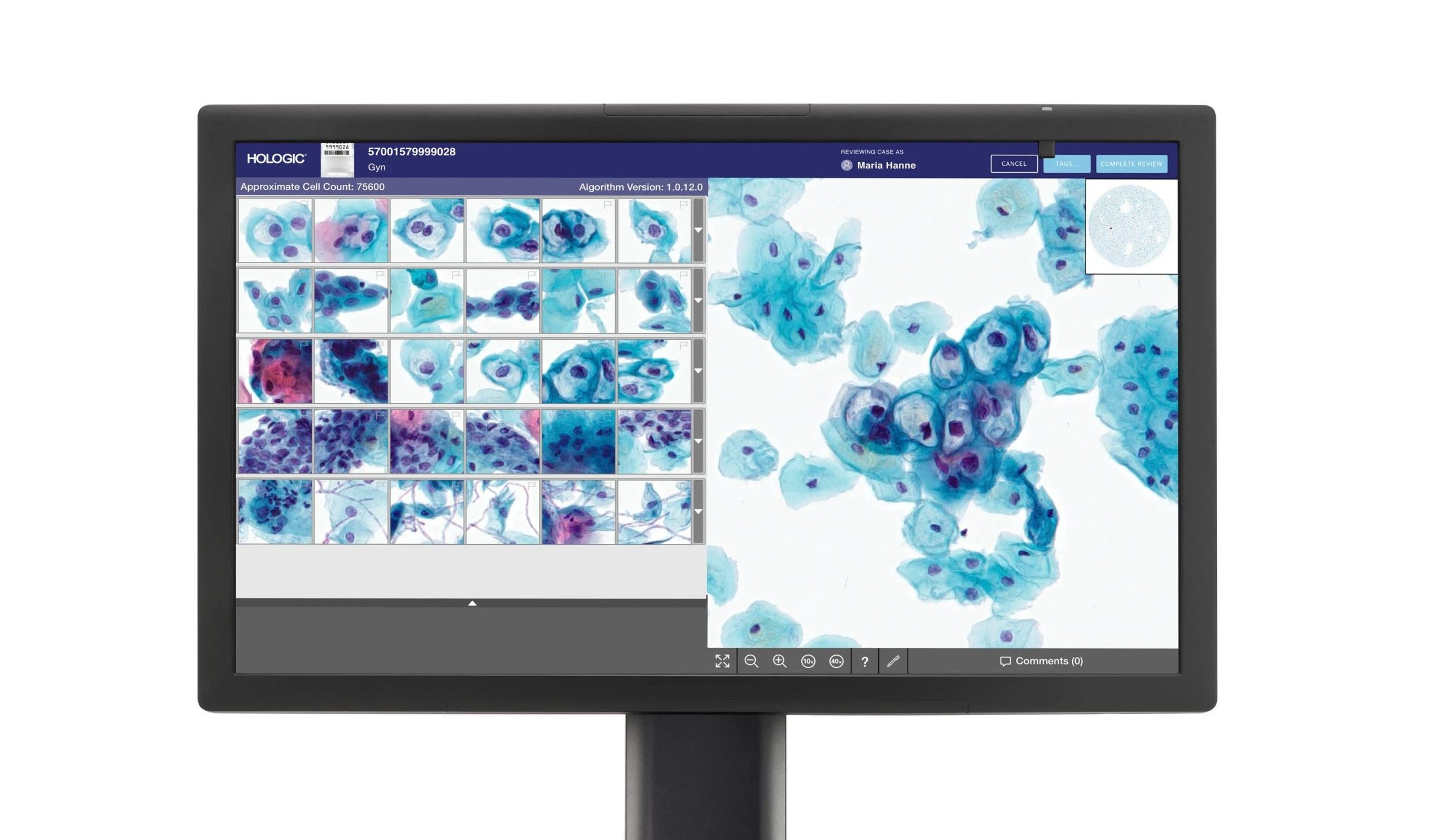
The Genius Digital Diagnostics System represents a breakthrough in medical diagnostics, particularly in the detection of cervical cancer cells and precancerous lesions. Traditional cervical cancer screenings involve Pap tests, where cervical cell samples are collected and analyzed on glass slides in a laboratory setting. However, Hologic’s system digitizes these glass slides, enabling analysis through its AI algorithm.
This digital cytology system enhances sensitivity without sacrificing specificity, leading to more accurate and timely detection of abnormalities. Hologic asserts that its system reduces false negatives by 28% for high-grade squamous intraepithelial lesions and more severe conditions compared to traditional microscopic review methods.
Moreover, the Genius Digital Diagnostics System offers numerous benefits to healthcare professionals. It enables remote case review, allowing experts from geographically dispersed locations to collaborate effectively. Additionally, the system is scalable, adapting to evolving laboratory needs. Its components include a digital imager for image acquisition, the Genius Cervical AI algorithm for analysis, an image management server for storage, and the Genius Review Station for case review.
The FDA approval marks a significant step forward for Hologic, as the company prepares for the commercial launch of its Genius Digital Diagnostics System later this year. While the technology is already available in Europe, Australia, and New Zealand, its availability in the U.S. will undoubtedly enhance cancer screening efforts and improve patient outcomes.
In a broader context, the integration of AI and machine learning technologies into healthcare applications continues to offer promising benefits. These technologies play a crucial role in early disease detection, treatment optimization, and healthcare management. For instance, AI is increasingly utilized in diabetes management and predicting Alzheimer’s disease progression.
Furthermore, AI is transforming the landscape of cancer research and drug development. Companies like Absci and pharmaceutical giant AstraZeneca are leveraging AI to accelerate the discovery of novel cancer therapies. As funding for AI-powered healthcare solutions increases, regulators and industry stakeholders grapple with the challenges of regulating and implementing these technologies effectively.
Discussions at events like the HIMSS AI in Healthcare Forum underscore the importance of embracing and investing in AI initiatives. Panelists at the forum deliberated on strategies to optimize the efficacy of AI in healthcare and the considerations involved in choosing between adopting existing AI solutions or developing new infrastructure.
As the healthcare industry continues to embrace AI-driven innovations, collaboration between stakeholders, including regulators, industry leaders, and healthcare professionals, will be essential to maximize the potential benefits of these transformative technologies. Hologic’s Genius Digital Diagnostics System exemplifies the potential of AI to revolutionize cancer screening and improve patient care.
The FDA approval of Hologic’s Genius Digital Diagnostics System underscores the transformative potential of AI in healthcare. By enhancing the sensitivity and accuracy of cervical cancer screening, this innovative system promises to revolutionize disease detection and improve patient outcomes. Moreover, its scalability and remote case review capabilities empower healthcare professionals to deliver more effective and timely interventions. However, realizing the full benefits of AI in healthcare requires strategic investments and thoughtful regulatory frameworks. Collaborative efforts between industry stakeholders, regulators, and healthcare professionals are essential to navigate the complexities of AI implementation and ensure its responsible and equitable deployment. As we embark on this AI-driven healthcare revolution, the Genius Digital Diagnostics System serves as a beacon of innovation and progress in the fight against cancer.

