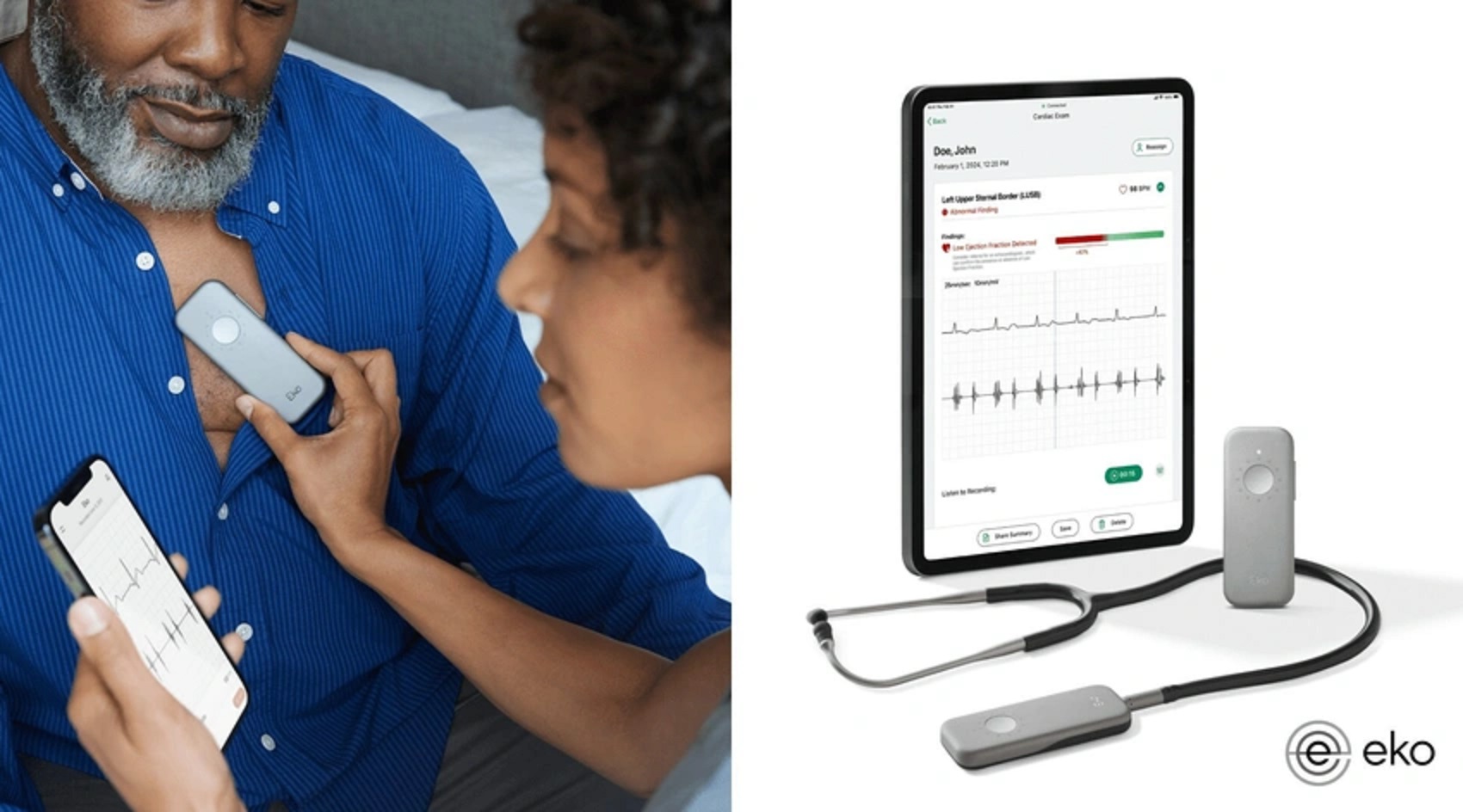
Eko Health celebrates a significant milestone with FDA 510(k) clearance for its AI-enabled cardiac tool, the Eko Low Ejection Fraction Tool (ELEFT). This groundbreaking innovation streamlines cardiac care by swiftly identifying low ejection fraction, a key indicator of heart failure, during routine examinations. Integrated seamlessly into Eko’s SENSORA Cardiac Early Detection Platform, ELEFT enhances diagnostic capabilities, offering comprehensive screening for atrial fibrillation and structural heart murmurs. The company’s commitment to advancing cardiac health is evident in its extensive portfolio of FDA-cleared solutions, reflecting a broader industry trend towards leveraging technology for improved patient outcomes.
Eko Health, a trailblazer in digital health solutions, achieves a significant breakthrough with FDA clearance for its AI-enabled cardiac tool. Designed to revolutionize cardiac care, the Eko Low Ejection Fraction Tool (ELEFT) empowers healthcare providers with rapid and accurate detection capabilities. With just 15 seconds using an Eko stethoscope, clinicians can now identify low ejection fraction, facilitating early intervention and treatment planning. This milestone underscores Eko Health’s commitment to innovation and its mission to enhance patient outcomes through cutting-edge technology. The integration of ELEFT into the SENSORA Cardiac Early Detection Platform further solidifies the company’s position as a leader in the tech-enabled cardiac health sector.
Eko Health, a digital health startup dedicated to revolutionizing cardiac and lung disease management, has achieved a significant milestone. The company proudly announces the FDA 510(k) clearance for its latest innovation: the AI-enabled cardiac tool. This groundbreaking tool, known as the Eko Low Ejection Fraction Tool (ELEFT), promises to revolutionize early detection in cardiac care by identifying low ejection fraction, a critical indicator of heart failure.
Eko Low Ejection Fraction Tool: Redefining Cardiac Care
ELEFT has been meticulously designed and rigorously tested to empower healthcare providers with swift and accurate detection capabilities. With just 15 seconds during a routine examination using an Eko stethoscope, providers can now identify low ejection fraction, facilitating early intervention and treatment planning. This innovative tool not only streamlines the diagnostic process but also enhances patient outcomes by enabling timely interventions.
Integration with SENSORA Cardiac Early Detection Platform
ELEFT seamlessly integrates with Eko’s esteemed SENSORA Cardiac Early Detection Platform, enhancing its existing suite of FDA-cleared algorithms. This integration bolsters the platform’s capabilities, allowing for comprehensive screening for conditions such as atrial fibrillation and structural heart murmurs. The addition of ELEFT represents a significant advancement in the platform’s ability to detect and manage a broad spectrum of cardiac issues.
Addressing a Pressing Healthcare Need
Heart failure poses a significant healthcare challenge, affecting millions of individuals worldwide. In the United States alone, over 6.2 million adults grapple with this condition, highlighting the urgent need for enhanced detection methods. Eko Health’s ELEFT emerges as a pivotal solution, empowering clinicians to identify patients at risk of heart failure during routine physical examinations. This proactive approach holds immense promise, particularly for underserved communities where access to advanced diagnostic tools may be limited.
Insights from Eko Health’s Leadership
Jason Bellet, the co-founder and COO of Eko Health, emphasizes the transformative impact of ELEFT on cardiac care. He underscores the significance of FDA clearance in expanding access to early detection, particularly in underserved communities. Bellet expresses gratitude to the FDA and development partner Mayo Clinic, recognizing their pivotal roles in advancing healthcare innovation.
The Broader Industry Landscape
Eko Health’s achievement underscores a broader trend within the tech-enabled heart health sector. The company’s success follows substantial funding injections, with $65 million secured in Series C funding in 2020, further augmented by a $30 million extension in 2022. Eko Health has consistently demonstrated its commitment to innovation, securing multiple 510(k) clearances for its groundbreaking technologies.
Pioneering Innovations in Cardiac Health
Eko Health’s portfolio of FDA-cleared solutions extends beyond ELEFT, encompassing the Eko Murmur Analysis Software and the Duo portable ECG and stethoscope. These innovations reflect the company’s dedication to advancing cardiac care through cutting-edge technology. Notable competitors in this space, such as CardioSignal and Ultromics, further underscore the growing emphasis on leveraging AI and machine learning for cardiac diagnostics.
Eko Health’s FDA clearance for the Eko Low Ejection Fraction Tool (ELEFT) marks a significant advancement in cardiac care. By leveraging AI technology, the company addresses a critical need for early detection, ultimately improving patient outcomes and reducing the burden of heart failure. The integration of ELEFT into the SENSORA Cardiac Early Detection Platform enhances diagnostic capabilities, offering clinicians a comprehensive solution for cardiac screening. As Eko Health continues to innovate and expand its portfolio of FDA-cleared solutions, it reaffirms its commitment to driving positive change in cardiac health management and transforming the future of healthcare.




