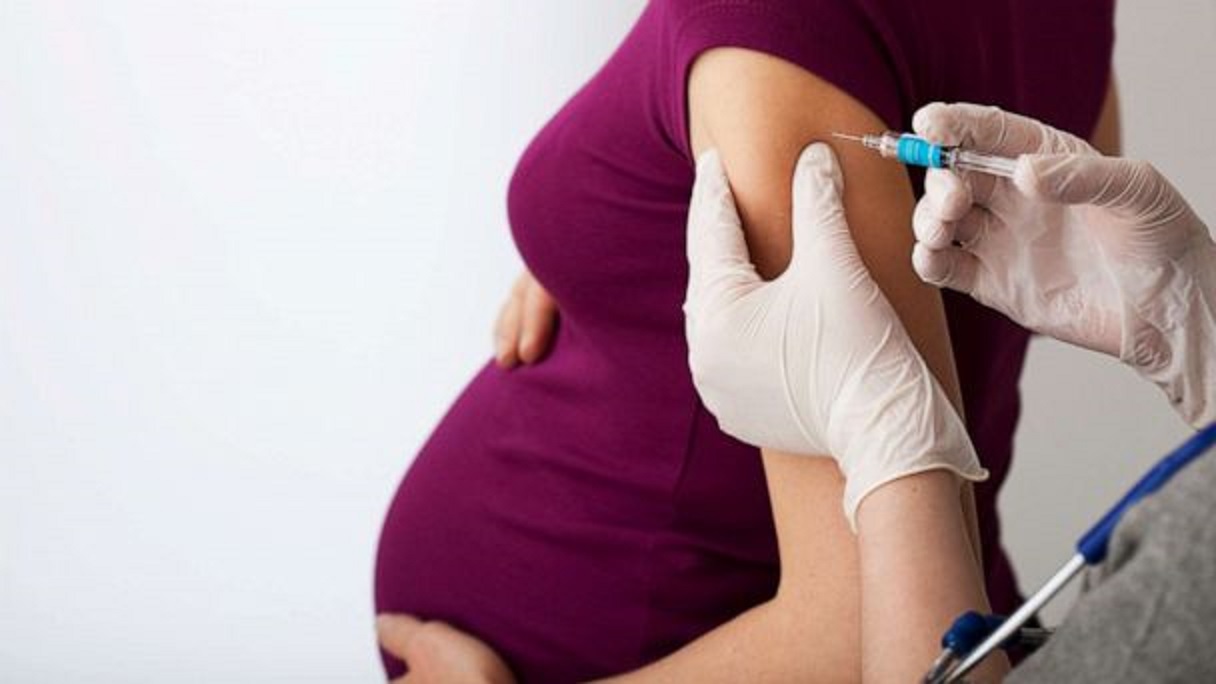
FDA has approved Pfizer’s Abrysvo, the first vaccine for gestational immunization, safeguarding infants up to 6 months from respiratory syncytial virus (RSV) ailments. Administered during weeks 32-36 of pregnancy, Abrysvo was proven effective in preventing RSV-induced lower respiratory tract diseases in infants. This milestone marks a significant advancement in maternal and infant healthcare, addressing RSV concerns and enhancing the well-being of vulnerable newborns.
The FDA has granted its endorsement to Pfizer’s Abrysvo (Respiratory Syncytial Virus Vaccine), marking a significant milestone as the inaugural vaccine sanctioned for active gestational immunization. This breakthrough vaccine is aimed at safeguarding infants from birth up to six months of age against lower respiratory tract disease (LRTD) and severe LRTD caused by respiratory syncytial virus (RSV).
Underlining its commitment to maternal and infant health, the FDA has approved for Abrysvo’s utilization during gestational weeks 32 through 36. This green light follows meticulous clinical investigations that have affirmed Abrysvo’s efficacy in averting both LRTD and severe LRTD prompted by RSV in infants born to mothers who received the vaccine during pregnancy. This pioneering development offers renewed hope for the prevention of RSV-related ailments in the vulnerable early stages of life.
Pfizer’s Abrysvo holds the distinction of being the maiden vaccine designed to address RSV concerns through active gestational immunization. With the FDA’s nod, a novel avenue in maternal and neonatal care opens up, potentially mitigating the burden of respiratory infections among infants. This decision reflects the intersection of rigorous research, medical innovation, and a commitment to enhancing the health and well-being of the most susceptible members of our population.




