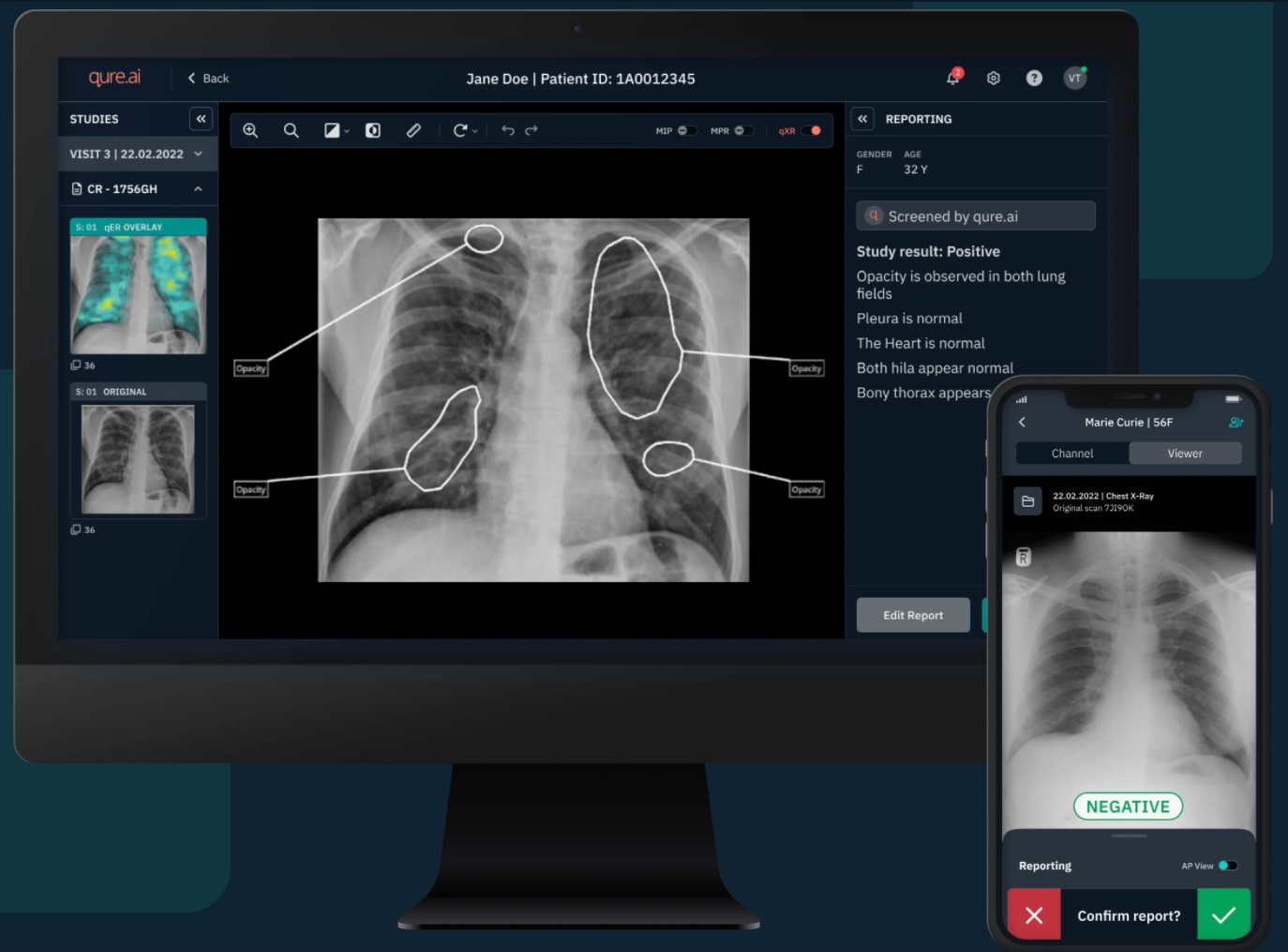
Qure.ai, a trailblazer in medical imaging solutions, celebrates its 13th FDA clearance with qXR-LN, an AI-powered tool for lung nodule detection. This pioneering technology signifies Qure.ai’s supremacy in AI-driven advancements for plain film radiography and medical imaging. The FDA clearance, the sixth for Qure’s chest X-ray solutions, validates qXR-LN’s capability in detecting and localizing lung nodules, targeting Radiologists, Pulmonologists, and ER physicians. The device’s pivotal studies affirm its efficacy in achieving a 94% detection rate. The introduction of qXR-LN stands as a remarkable leap in fighting lung cancer, addressing the crucial need for early detection among non-smokers. Qure.ai’s commitment reshapes healthcare standards, propelling AI’s transformative role in patient care.
Qure.ai, a prominent figure in medical imaging solutions, has recently achieved its 13th FDA clearance for its AI-powered technologies. This milestone comes with the approval of qXR-LN, a cutting-edge AI-driven solution specifically designed to detect and pinpoint lung nodules on chest X-rays. This achievement solidifies Qure.ai’s status as a trailblazer in advancing AI-driven innovations for plain film radiography and medical imaging. Notably, this clearance marks the sixth for Qure’s chest X-ray solutions, standing out as the lone FDA-cleared solution using computer vision for detecting and localizing lung nodules, tailored for use by Radiologists, Pulmonologists, and ER physicians. The introduction of qXR-LN heralds a significant opportunity to identify potentially malignant pulmonary nodules, a critical step in the battle against lung cancer.
This pioneering technology, qXR-LN, is a state-of-the-art computer-aided detection software engineered to identify and highlight areas suggestive of suspected pulmonary nodules measuring 6 to 30 mm in size. Tailored for the incidental adult population, this innovative device represents a game-changer in diagnostic technology. Moreover, it serves as a crucial second reader for physicians, aiding in the analysis of adult frontal (AP/PA) chest radiographs obtained through digital radiographic systems.
Prashant Warier, Co-Founder and CEO of Qure.ai emphasized the company’s commitment to the US healthcare landscape. He highlighted the significance of this clearance as a pivotal leap in combating lung cancer and reiterated the company’s dedication to revolutionizing healthcare through innovative solutions. This FDA clearance reaffirms Qure.ai’s mission to enhance early cancer detection, ultimately improving patient outcomes and marking a significant stride in the fight against this lethal disease.
To establish the safety and effectiveness of qXR-LN, Qure conducted two pivotal studies. In the initial study, the device demonstrated standalone performance meeting predefined success criteria, achieving an impressive 94% Area Under the Curve (AUC) for nodule detection. This study incorporated chest X-ray scans collected from various states and sites across the United States, assessed against ground truth determined by five American board-certified Radiologists.
The second pivotal study involved a multi-reader, multi-case clinical evaluation demonstrating qXR-LN’s enhancement in pulmonary nodule detection across different reader groups, including radiologists, pulmonologists, and emergency room physicians. Significantly, some non-radiologist groups approached or even surpassed the baseline performance of radiologists when aided by qXR-LN.
Lung cancer stands as the leading cause of cancer-related deaths in the US, with late-stage diagnoses resulting in alarmingly low survival rates. A significant portion of lung cancer cases occurs in individuals who have never smoked or smoked minimally, often overlooked by conventional screening programs, leading to delayed diagnoses. Early identification of lung nodules on plain film radiography is pivotal for timely interventions, treatment planning, and disease monitoring, contributing to more efficient and effective healthcare.
Dr. Vishisht Mehta, Director of Interventional Pulmonology at Comprehensive Cancer Centers of Nevada, underscored the critical role of AI-driven tools like qXR-LN in early-stage lung cancer detection. He emphasized the transformative impact of AI algorithms in pulmonary imaging, particularly within oncology.
Beyond lung nodule detection, Qure.ai’s portfolio spans diverse applications reshaping healthcare. These FDA-cleared innovations range from assessing Cardiomegaly (Heart Failure) to aiding in tube placement and triaging critical cases like Pneumothorax and Pleural Effusion from Chest X-rays. Additionally, their applications extend to acute intracranial hemorrhage detection, providing volumetric bleed measurement, cranial fracture detection, and maximal midline shift quantification from non-contrast head CT scans.
Dr. Mannudeep Kalra, a Radiologist at Massachusetts General Hospital and associate Professor of Radiology at Harvard Medical School, highlighted the role of AI algorithms in enhancing radiology workflow and accuracy. He emphasized the importance of AI-driven solutions like qXR-LN in improving patient outcomes, aligning with their internal evaluations.




