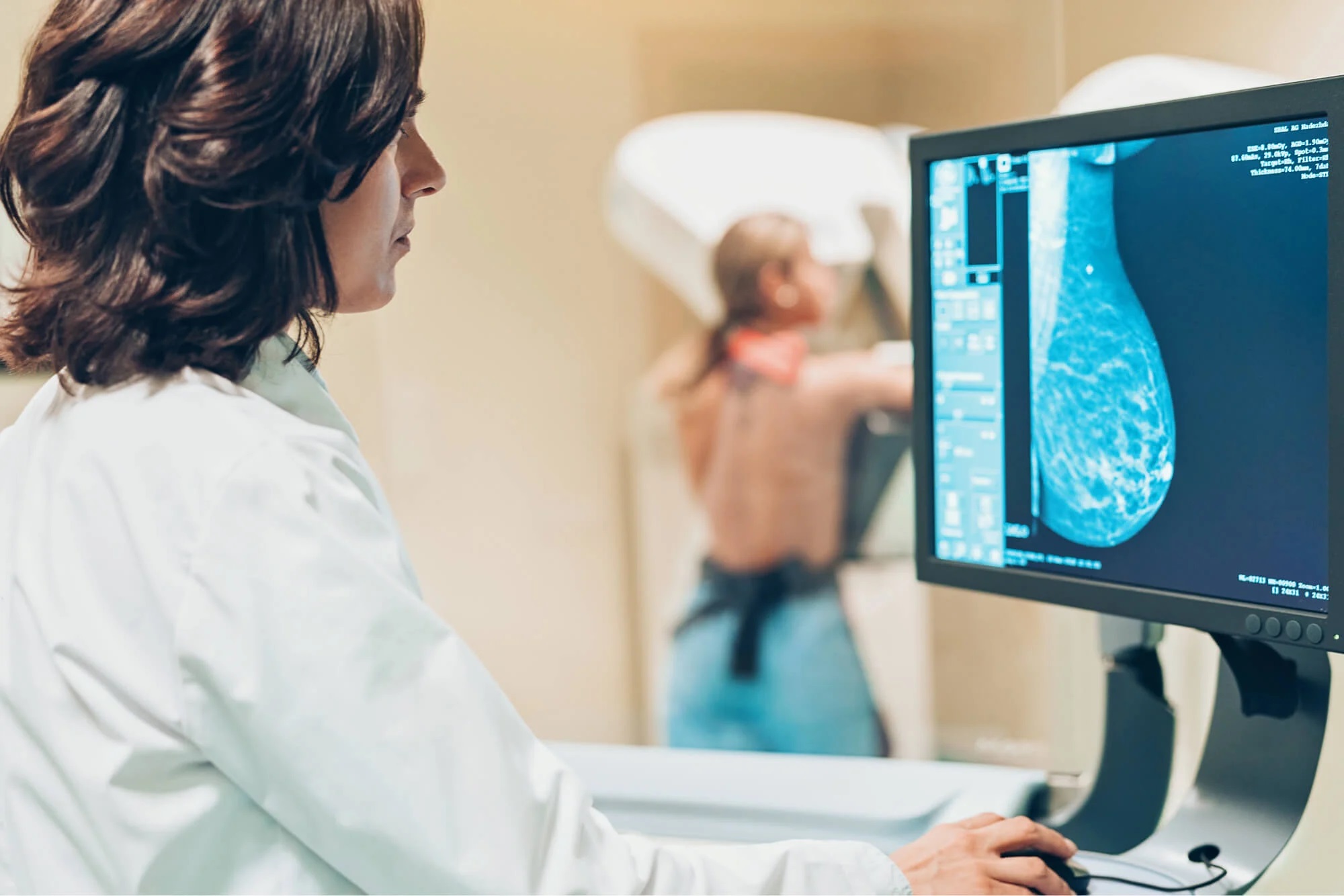
Discover how Transpara Breast AI by ScreenPoint Medical revolutionizes mammography screening by providing radiologists with advanced support in cancer detection. Through real-world clinical studies presented at the European Congress of Radiology, learn how Transpara significantly improves cancer detection rates while reducing recall rates. Explore the findings from randomized controlled trials and prospective studies, showcasing the effectiveness of AI-driven triaging in streamlining workload without compromising diagnostic accuracy. With FDA clearance and CE Mark approval, Transpara offers a globally recognized solution for enhancing breast cancer screening outcomes.
ScreenPoint Medical is set to unveil its cutting-edge Transpara Breast AI at the prestigious 2024 European Congress of Radiology (ECR) from February 28 to March 3, 2024, situated at Booth #AI-31 in Expo Hall X1. Transpara represents a groundbreaking advancement in mammography screening, providing radiologists with an invaluable “second pair” of eyes to enhance cancer detection rates and minimize recall rates.
The efficacy of Transpara in real-world clinical settings and its seamless integration into workflow processes have been extensively demonstrated through a myriad of global practice and clinical research endeavors. At ECR 2024, four presentations and three posters will elucidate these clinical and workflow benefits:
1. MASAI Trial Analysis: In a pioneering randomized controlled trial, researchers delved into cancer detection rates and the spectrum of identified cancers within the comprehensive MASAI-trial study cohort. The incorporation of AI into screening yielded noteworthy enhancements compared to conventional double-reading approaches. These results will be discussed in detail during the presentation titled “Cancer detection about type and stage in the randomized Mammography Screening with Artificial Intelligence trial (MASAI)” (RPS-2002, ACV Research Stage 2, March 2, 2 pm).
2. AITIC Trial Findings: Another prospective clinical trial scrutinized the potential of AI in streamlining workload by triaging low-risk cases for direct AI assessment while allocating human reading resources to higher-risk scenarios. This presentation, titled “Is it worth reading low-risk breast cancer screening mammograms as determined by an artificial intelligence (AI) system? A prospective, population-based study for DM and DBT (AITIC trial)” (RPS-1202, ACV Research Stage 1, March 1, 8 am), underscores the substantial reduction in reading workload facilitated by AI-driven triaging without compromising diagnostic accuracy.
Additional presentations encompass:
– “How much has AI improved over the last five years? A benchmark evaluation of different versions of an AI mammography interpretation system” (RPS-1202, ACV Research Stage 1, March 1, 8 am).
– “Are AI-detected interval cancers actionable for recall in a real screening setting? An informed review of 120 interval cancer cases with high AI scores in breast screen Norway (RPS-2405, ACV Research Stage 1, March 3, 11.30 am).
E-posters will delve into topics such as:
– “Enhancing Mammography Screening Sensitivity with AI-Assistance: Evidence from a Vietnamese Study Cohort.”
– “AI breast cancer detection as a decision-support tool in mammography: what is the added value in a clinical population?”
– “Using AI to automatically compute volumetric breast density and BIRADS density grade in mammography and breast tomosynthesis images.”
Mark Koeniguer, CEO of ScreenPoint Medical, emphasized, “We are delighted that both users and researchers continue to recognize the value of Transpara in enhancing the mammography screening process. It is imperative to highlight the global consistency in Transpara’s performance, as evidenced by a spectrum of retrospective, prospective, and randomized controlled trials, all affirming its efficacy in facilitating early cancer detection while maintaining stable recall rates. Women should not have to compromise on the quality of their breast screenings.”
Transpara holds FDA clearance and European regulatory approval (CE Mark) for both abnormality detection and density assessment across 2D and 3D mammography platforms from various manufacturers. Deployed across hundreds of leading centers spanning over 30 countries, Transpara seamlessly collaborates with radiologists, significantly enhancing screening outcomes while optimizing reading workflows. Research indicates that Transpara can potentially detect up to 45% of interval cancers earlier, thereby underscoring its pivotal role in augmenting screening efficacy.
The journey towards more effective breast cancer screening takes a significant leap forward with Transpara Breast AI. Through a culmination of groundbreaking research, this AI-powered solution showcases remarkable potential in improving cancer detection rates while minimizing recall rates. As highlighted in the presentations and posters at the European Congress of Radiology, Transpara’s impact resonates globally, offering radiologists a reliable ally in the fight against breast cancer. With regulatory approvals and a growing adoption worldwide, Transpara heralds a new era of precision and efficiency in mammography screening, ultimately empowering healthcare providers to deliver enhanced care to women worldwide.

