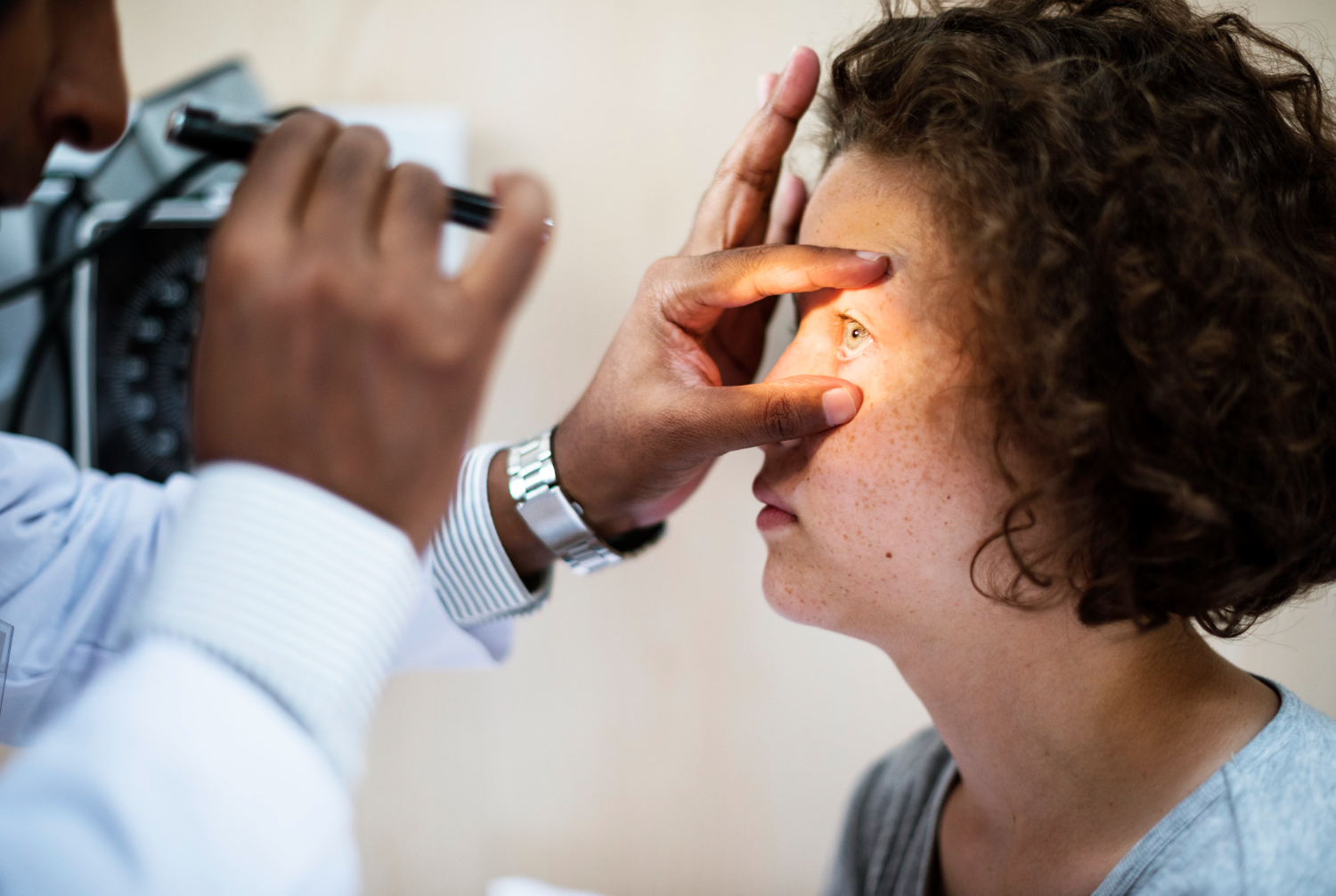
Understanding the Eye Risk Concerns
Recent findings have raised alarming concerns about potential ophthalmic complications associated with popular diabetes medications semaglutide and tirzepatide. These incretin-based therapies, while highly effective for managing type 2 diabetes and obesity, are now under scrutiny for their possible connection to vision-related side effects.
Critical Study Findings and Patient Cases
A groundbreaking case series involving nine patients has revealed significant ocular complications potentially linked to these medications. The most concerning finding was Nonarteritic Anterior Ischemic Optic Neuropathy (NAION), documented in seven patients, resulting in sudden vision loss due to optic nerve infarction. Additional cases included bilateral papillitis and paracentral acute middle maculopathy, both serious conditions affecting vision.
Understanding the Biological Mechanism
Scientists hypothesize that rapid blood sugar correction may be the underlying cause of these complications. Patients experienced dramatic decreases in HbA1c levels, dropping from 8.4%-10.2% to 5.5%-6.7% after treatment initiation. This substantial improvement in glycemic control might create ischemic stress on optic nerve and retinal tissues, potentially triggering these vision-related complications.
Market Impact and Industry Response
The widespread adoption of these medications is significant, with nearly 2% of the U.S. population receiving semaglutide prescriptions in 2023. The drug’s market presence is substantial, generating $38 billion in pharmaceutical expenditures and showing 100% growth compared to the previous year. This extensive use emphasizes the crucial need for comprehensive safety monitoring.
Professional Guidelines and Safety Measures
The American Academy of Ophthalmology has issued clear guidelines recommending immediate treatment cessation and medical attention for patients experiencing vision changes. Healthcare providers are now developing enhanced screening protocols, particularly for patients with pre-existing eye conditions like diabetic retinopathy.
Future Recommendations and Safety Protocols
Moving forward, implementing robust postmarketing surveillance studies is essential to better understand these complications. Healthcare providers must prioritize patient education about potential vision-related symptoms and establish clear communication channels between pharmaceutical companies, healthcare providers, and regulatory bodies to optimize patient safety and treatment outcomes.
Discover the latest Provider news updates with a single click. Follow DistilINFO HospitalIT and stay ahead with updates. Join our community today!




Leave a Reply